Abstract
Figure legends:
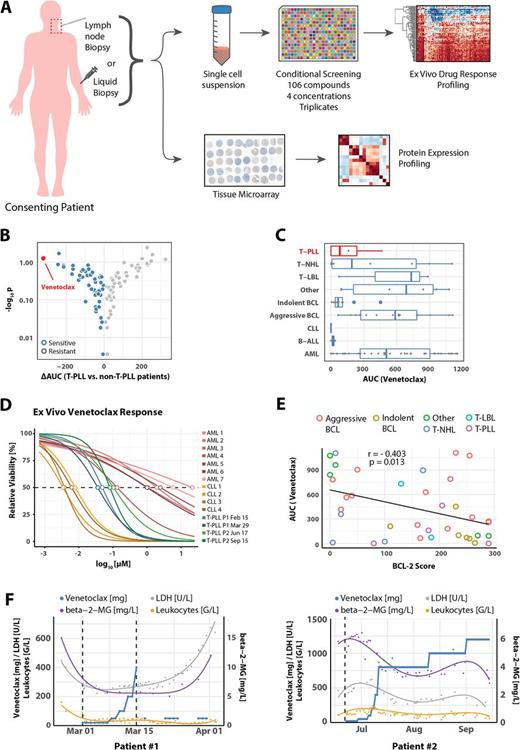
A) Lymph node and liquid biopsies were taken from consenting patients and used for expression profiling via tissue microarrays (TMA) and ex vivo drug response profiling (n = 86).
B) Mean differential areas under the curve (AUCs) were plotted for individual compounds comparing ex vivo effects in T-PLL versus non-T-PLL samples (n = 106).
C) AUCs of Venetoclax in individual ex vivo samples stratified by indication (n = 86).
D) High resolution dose-response-curve of Venetoclax of CLL, T-PLL, and AML samples. Concentrations ranging from 13µM to 0.7 nM in three-fold dilutions at 10 concentration points with 8 replicates each.
E) AUCs of Venetoclax versus corresponding BCL-2 expression scores for individual patients.
F) Venetoclax ex vivo response matches clinical response in two T-PLL patients.
T-cell prolymphocytic leukemia (T-PLL) is an aggressive T-lymphoid malignancy usually refractory to current treatment strategies and associated with a short overall survival. Clinicians have a large armamentarium of anticancer drugs, however therapeutic progress in T-PLL is lacking, mainly because it is extremely rare and thus challenging to perform studies and to select effective treatments.
By applying next-generation functional testing of primary patient-derived lymphoma cells using a library of 106 FDA-approved anti-cancer drugs or compounds currently in clinical development we pursued to identify novel effective treatments for T-PLL patients (Figure Panel A).
We found that the BCL-2 inhibitor venetoclax (ABT-199) demonstrated the strongest T-PLL specific response when comparing individual ex vivo drug response in 86 patients with refractory hematologic malignancies (Figure Panel B). Comparing disease entities, we also observed strong responses to venetoclax in chronic lymphatic leukemia (CLL) and varying responses in aggressive lymphoma and acute myeloid leukemia (AML) in line with reported clinical responses and which we could confirm by high resolution dose responses (Figure Panel C, D). Mechanistically, responses to venetoclax correlated with protein expression of BCL-2 but not with expression of the BCL-2 family members MCL-1 and BCL-XL in lymphoma-cells (Figure Panel E). BCL-2 expression was inversely correlated with the expression of MCL-1. Based on the ex vivo responses venetoclax treatment was commenced in two late stage refractory T-PLL patients resulting in striking clinical responses as evidenced by remission in lymphadenopathy, splenomegaly, lymphocytosis, and serum-levels of LDH and b2-Mig (Figure Panel F).
Our findings demonstrate first time evidence of single agent activity of venetoclax both, ex vivo and in human offering a novel agent in T-PLL.
Hoermann: Gilead: Honoraria, Research Funding; Amgen: Honoraria; Ariad: Honoraria; Novartis: Honoraria. Sperr: Amgen: Consultancy, Honoraria, Research Funding; Meda: Research Funding; Teva: Honoraria; Phadia: Research Funding; Celgene: Consultancy, Honoraria; Novartis: Other: Register. Valent: Pfizer: Honoraria; Blueprint: Research Funding; Ariad: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Honoraria; Teva: Honoraria; Novartis: Honoraria, Research Funding; Deciphera: Honoraria, Research Funding; Incyte: Honoraria. Jaeger: Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Staber: MSD: Honoraria; Gilad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Abbie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal